Fixed Drug Eruptions
- Author: David F Butler, MD; Chief Editor: Dirk M Elston
Background
Adverse reactions to medications are common and often manifest as a cutaneous eruption.
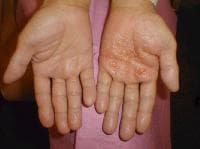
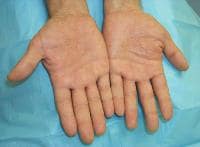
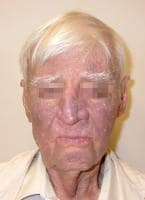
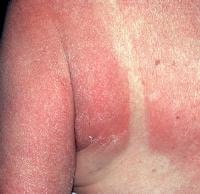
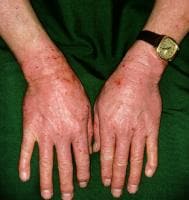
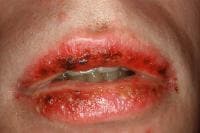
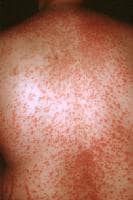
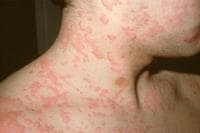
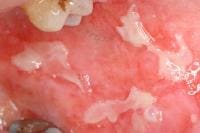
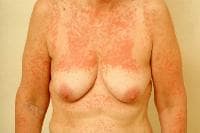
Drug-induced cutaneous disorders frequently display a characteristic clinical morphology such as morbilliform exanthem, urticaria, hypersensitivity syndrome, pseudolymphoma, photosensitivity, pigmentary changes, acute generalized exanthematous pustulosis, lichenoid dermatitis, vasculitis, Stevens-Johnson syndrome, or fixed drug eruption (FDE). The term fixed drug eruption describes the development of one or more annular or oval erythematous patches as a result of systemic exposure to a drug; these reactions normally resolve with hyperpigmentation and may recur at the same site with reexposure to the drug. Repeated exposure to the offending drug may cause new lesions to develop in addition to "lighting up" the older hyperpigmented lesions.
Several variants of fixed drug eruption have been described, based on their clinical features and the distribution of the lesions.[1, 2, 3, 4, 5, 6] These include the following:
- Pigmenting fixed drug eruption
- Generalized or multiple fixed drug eruption
- Linear fixed drug eruption
- Wandering fixed drug eruption
- Nonpigmenting fixed drug eruption
- Bullous fixed drug eruption
- Eczematous fixed drug eruption
- Urticarial fixed drug eruption
- Erythema dyschromicum perstans–like fixed drug eruption
- Vulvitis
- Oral
- Psoriasiform
- Cellulitislike eruption[7]
Pathophysiology
Although the exact mechanism is unknown, recent research suggests a cell-mediated process that initiates both the active and quiescent lesions. The process may involve an antibody-dependent, cell-mediated cytotoxic response.[8] CD8+effector/memory T cells play an important role in reactivation of lesions with re-exposure to the offending drug.[9, 10]
The offending drug is thought to function as a hapten that preferentially binds to basal keratinocytes, leading to an inflammatory response.[11] Through liberation of cytokines such as tumor necrosis factor-alpha, keratinocytes may locally up-regulate expression of the intercellular adhesion molecule-1 (ICAM1).[12] The up-regulated ICAM1 has been shown to help T cells (CD4 and CD8) migrate to the site of an insult.[13, 14]
The newly arriving and residential CD8 cells likely perpetuate tissue damage by their production of the inflammatory cytokines interferon-gamma and tumor necrosis factor-alpha. CD8 cells isolated from active lesions have also been shown to express alpha E beta 7, a ligand for E-cadherin, which may further contribute to the lymphocyte’s ability to localize to the epidermis. Other cell surface molecules, such as CLA/alpha4beta1/CD4a, that bind E-selectin/vascular cellular adhesion molecule-2/ICAM1 help to further attract CD8 cells to the area.[8]
Changes in cell surface markers allow vascular endothelium to select CD4 cells for migration into active lesions. These regulatory CD4 cells likely produce interleukin 10, which has been shown to help suppress immune function, resulting in a resting lesion.[8] As the inflammatory response dissipates, interleukin 15 expression from keratinocytes is thought to help ensure the survival of CD8 cells, helping them fulfill their effector memory phenotypes. Thus, when reexposure to the drug occurs, a more rapid response develops in the exact location of any prior lesions.[8]
Epidemiology
Frequency
United States
The prevalence of drug eruptions has been reported to range from 2-5% for inpatients and greater than 1% for outpatients.[15] Fixed drug eruptions may account for as much as 16-21% of all cutaneous drug eruptions. The actual frequency may be higher than current estimates, owing to the availability of a variety of over-the-counter medications and nutritional supplements that are known to elicit fixed drug eruptions.
International
The international prevalence is variable but is likely similar to that in the United States. Most studies report fixed drug eruptions to be the second or third most common skin manifestation of adverse drug events.[16]
Mortality/Morbidity
Widespread lesions may initially mimic toxic epidermal necrolysis, but they have a benign clinical course.[17] Localized hyperpigmentation is a common complication, but pain, infection, and, rarely, hypopigmentation, also may occur.[1]
Race
Fixed drug eruptions have no known racial predilection. A genetic susceptibility to developing a fixed drug eruption with an increased incidence of HLA-B22 is possible.[18, 19]
Sex
One large study of 450 patients revealed a male-to-female ratio of 1:1.1 for fixed drug eruptions.[1]
Age
Fixed drug eruptions have been reported in patients as young as 1.5 years and as old as 87 years. The mean age at presentation is 30.4 years in males and 31.3 years in females.[1]